 |
| Module 3: Toxicology - Section 2: Classification of hazardous substances |
| TOX 2.3: Lecture: Classification systems for hazardous substances |
 |
| Module 3: Toxicology - Section 2: Classification of hazardous substances |
| TOX 2.3: Lecture: Classification systems for hazardous substances |
In order to make sense of the large number of hazardous chemical compounds or used in industry and their effects, we need a way of classifying them. Classification reduces a large amount of data to manageable proportions and is also a way of remembering the important features of these chemical compounds.
(As from occupational hygiene)
Avoid using the term "fumes" when you mean vapour, gas or even smoke. Be precise in your description when you take notes, write reports or communicate, as each term has different implications with regard to source, control and impact.
Mucous membranes, skin, eyes, upper respiratory tract, lower respiratory tract.
Many, probably most, complaints among workers working with chemical substances fall into this category, and may interfere with quality of life. Irritative symptoms may also be the first indication of excessive exposure. Although it must have a specific pathophysiological basis, irritation is often used as a non-specific concept. It is sometimes contrasted with allergy, as in irritative vs allergic contact dermatitis.
Very large category. See classification by organ system. Examples include:
Hepatotoxins - tetrachloroethane
Neurotoxins (peripheral or central) - mercury, lead, solvents, pesticides
Nephrotoxins - heavy metals
Haematotoxins - benzene
Cardiotoxins - carbon disulfide
Reproductive toxins - glycol ethers, lead
Rapidly penetrate blood brain barrier and cause CNS excitation or depression - organic solvents.
May be critical to cell, or occur in gamete (sperm or ovum).
May be basis of carcinogenesis.
Although a number of substances are mutagenic in the laboratory, ionizing radiation is the best known mutagenic hazard in humans.
A number of substances are teratogenic in animal experiments, e.g. a number of pesticides. However, extrapolation to humans is difficult. The best known human example is (environmental) methyl mercury. (Look up: Minamata disaster). Epidemiology difficult as need to be able to study critical period of foetal development of 1 - 5 weeks to establish causation.
Initiators: primary - cancer at point of contact (e.g. alkylating agents)
Procarcinogens: metabolised into carcinogen (e.g. benzidine)
Promoters: Co-carcinogens - accelerate tumour growth (may be long after initiation) (E.g. in the case of asbestos and lung cancer, there has been debate as to whether it is a "complete carcinogen" or a promoter which needs an initiator co-factor).
Dusts with high capacity of causing pneumoconiosis: asbestos, silica, coal
"Nontoxic" dusts - low capacity to cause pneumoconiosis, although may do so, but can all irritate respiratory tract, e.g. clay, mica, limestone, portland cement.
Question 1: What is the distinction between inorganic and organic?
Answer.
Halogens: chlorine, bromine, fluorine
Highly reactive « thus part of many compounds as well as toxic on their own as gases with strong respiratory tract irritant properties.
Acids, alkalis: sulphuric acid; ammonia, calcium oxide
Depending on solution, can be strong skin and respiratory tract irritants (hydrofluoric acid, e.g, causes serious acid burns).
- Oxides of nitrogen (NOX): nitrogen (N2), nitrogen dioxide (NO2), nitrous oxide (N2O), nitric oxide (NO);
- carbon monoxide (CO), carbon dioxide (CO2);
- sulphur dioxide SO2, hydrogen sulphide (H2S)
Metals:
Malleable, ductile, metallic lustre.
Heavy metals: lead, mercury, arsenic, cadmium
Hard metals: cobalt, tungsten (and its carbide).
Other noxious gases: Arsine (AsH3) - Haemolytic anaemia
Carbon disulfide: (Inorganic solvent)
- Used in viscose rayon production, cellophane
- Multisystem toxin: eye lesions, hearing loss, coronary artery disease, reproductive defects, nervous system abnormalities.
These are covered in more detail in the section on Organic Solvents: HT: Solvents: review of general properties, HT Solvents: review of general effects
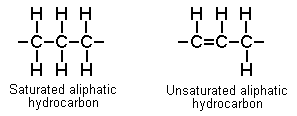
Aliphatic: (straight chain) hydrocarbons
Saturated (alkanes or "paraffins"): all carbon-carbon bonds are single.
E.g. methane, propane, n-hexane
- Simple asphyxiants on inhalation, skin irritants on contact
- Higher (longer) forms: irritants, CNS depression
Unsaturated (alkenes, alkynes): one or more carbon-to-carbon bonds is double
E.g., ethylene, isoprene: Simple asphyxiants, weak anaesthetics
Alicyclic hydrocarbons (alkanes or alkenes in ring structure)
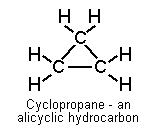
E.g. cyclopropane, cyclohexane: effect resembles that of aliphatics
Aromatic hydrocarbons (one or more "benzene rings")
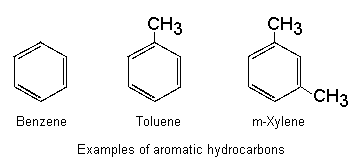
- Very important group: benzene, toluene ("toluol"), xylenes ("xylol")
- Irritant, anaesthetic properties greater than aliphatics
Question 2: Where is the general public likely to encounter benzene?
Answer
 Halogenated hydrocarbons: hydrocarbon chain plus a halogen:
Halogenated hydrocarbons: hydrocarbon chain plus a halogen:
Also very important group: occupationally and environmentally.
Question 3: Why environmentally?
Answer
E.g. Carbon tetrachloride; trichloroethylene; perchloroethylene
Respiratory and skin effects, Hepatotoxic, nephrotoxic, neurotoxic, carcinogenic?, teratogenic?
 Alcohols: hydrocarbon chain plus an hydroxyl group:
Alcohols: hydrocarbon chain plus an hydroxyl group:
E.g. methanol, ethanol, isopropyl alcohol
- Higher forms more toxic
- Methanol: very specific optic nerve toxicity on ingestion (on inhalation?)
By far the biggest problem from this category that you will encounter in the workplace is the effects of ethanol ingestion. You should know its toxic effects, both acute and chronic, in order to be able to treat these.
 Glycols: hydrocarbon chain with two hydroxyl groups
Glycols: hydrocarbon chain with two hydroxyl groups
E.g. ethylene glycol: antifreeze agent, low toxicity
 Phenols: benzene ring plus hydroxyl group
Phenols: benzene ring plus hydroxyl group
E.g. phenol - used in laboratories, including anatomy
- Potent irritant, cytotoxic
- Hydroquinone - allergic dermatitis
 Ketones: hydrocarbon chain plus a carbonyl (C=O ) group:
Ketones: hydrocarbon chain plus a carbonyl (C=O ) group:
-Very strong odours (no necessary correlation with toxicity as many people think)
E.g. acetone
- Methyl ethyl ketone (MEK)
- Methyl n-butyl ketone - one of the few substances causing peripheral neuropathy.
 Ethers: two hydrocarbon chains linked by an oxygen:
Ethers: two hydrocarbon chains linked by an oxygen:
(i) Simple ethers: e.g. ethyl ether - anaesthetics
(ii)Glycol ethers - multisystem toxicity including male reproductive system, teratogenesis
(iii)Cyclic ethers - used in epoxy resins, allergenic
(iv)Glycidyl ethers - sensitisers, reproductive toxins
 Esters: organic acid and alcohol
Esters: organic acid and alcohol
E.g. methanol and acetic acid = methyl acetate
- Strong odour
- Anaesthetics
 Aliphatic amines: aliphatic hydrocarbon plus amino group:
Aliphatic amines: aliphatic hydrocarbon plus amino group:
E.g. Methylamine (gas), diethylene triamine
- Derived from ammonia
- Irritant, allergenic
 Aromatic amines: benzene ring plus amino group
Aromatic amines: benzene ring plus amino group
Aniline, naphthylamine, benzidine
- Wide spectrum of toxicity, including:
 Nitro esters (nitric acid esters of aliphatic alcohols)
Nitro esters (nitric acid esters of aliphatic alcohols)
E.g. Nitroglycerin, ethylene glycol dinitrate
- Important in South Africa: explosives manufacture
- Vascular and cardiac effects, including headache, angina, sudden death from heart attack.
Question 4: It was said that explosive factory workers used to wipe a small amount of substances on the insides of their hats when they went off for the weekend. Why?
Answer
 Nitrosamines: contain a nitrosyl group:
Nitrosamines: contain a nitrosyl group:
-Occupational and environmental exposure (tobacco, foods, in vivo formation)
- Hepatotoxic; carcinogenic in animals and probably humans.
Formaldehyde
- Widespread use in fabrics (including clothing) and building materials - very common (indoor ) exposure
- Preservative, e.g. in anatomy
- Irritant (although also listed as cause of asthma), skin allergen, potential carcinogen
Polychlorinated biphenyls (PCBs)
- Electrical industry
- Notorious because of environmental contamination - persistent organic pollutant
- Causes: Yusho disease, chloracne, birth defects.
Dioxins
E.g. 2,3,7,8, tetrachlorodibenzo-p-dioxin (2,3,7,8 - TCDD)
- Contaminant in manufacture of phenoxy acid herbicides (including Agent Orange used to defoliate Viet Nam)
- Contaminant in incinerator ash and in burning of PVC - hence environmental concern
- Extremely toxic in lab
- Acute exposure - mutlisystem toxicity
- Chronic: chloracne, soft tissue sarcoma, ? reproductive effects
 Polycyclic aromatic hydrocarbons (PAHs)
Polycyclic aromatic hydrocarbons (PAHs)
E.g.: benzo(a)pyrene. (Besides being a by product in industry, it is used in the laboratory as an experimental carcinogen).
Coal tars (by product of coal conversion into coke): coal tar pitch, creosote
- PAHs are found in ambient air pollution from industry.
- Photodermatitis
- Cancer: Scrotal (chimney sweeps), lung.
Thermoplastics: can be reshaped by heat and pressure
Vinyl chloride The monomer is a gas: hepatic angiosarcoma, cirrhosis, acro-osteolysis
Polyvinyl Chloride (PVC): pneumoconiosis (if inhaled as fine dust)
Styrene monomer: irritant, asthma, CNS effects
Polystyrene ?
Thermosets: permanent cross-linking which is heat resistant
Isocyanates
- Monomer or dimer: potent sensitizers skin and respiratory tract, e.g. toluene diisocyanate (TDI)
- Polyurethane: foam, surface coatings.
Resins: formaldehyde or amino resins
E.g. urea - formaldehyde particle board foams
- Headaches, respiratory tract, skin irritation (occupational and residential)
Answer to Question 1:An organic substance is a carbon containing substance based on a hydrocarbon building block. (Back to main text).

Postgraduate Diploma in Occupational Health (DOH) - Modules 3: Occupational Medicine & Toxicology (Basic) by Profs Mohamed Jeebhay and Rodney Ehrlich, Health Sciences UCT is licensed under a Creative Commons Attribution-Noncommercial-Share Alike 2.5 South Africa License. Major contributors: Mohamed Jeebhay, Rodney Ehrlich, Jonny Myers, Leslie London, Sophie Kisting, Rajen Naidoo, Saloshni Naidoo. Source available from here. For any updates to the material, or more permissions beyond the scope of this license, please email healthoer@uct.ac.za or visit www.healthedu.uct.ac.za.
Last updated Jan 2007.
Disclaimer note: Some resources and descriptions may be out-dated. For suggested updates and feedback, please contact healthoer@uct.ac.za.